ABSTRACT-The lower Cambrian problematicum Yunnanozoon lividum, currently known from the Maotianshan Shale Member of the Yu’anshan Formation (Chengjiang fauna), is described and illustrated. This soft-bodied organism has been variously interpreted as a metazoan of unknown affinities (Hou et al., 1991), a chordate (Chen et al., 1995, 1996), or a hemichordate (Shu et al., 1996a). Shu et al. (1996b) recently interpreted a dorsally compacted specimen from Chengjiang as a new chordate, called Cathaymyrus diadexus. However, the anatomical resemblance with Yunnanozoon lividum raises questions regarding the validity of this taxon.
A chordate affinity for Y. lividum is supported by the presence in most specimens of a notochord (preserved as a pair of axial lines) and a region showing segmented muscles. The hemichordate hypothesis (Shu et al., 1996a) is based primarily on misinterpretation of the two axial lines as a gut, misinterpretation of muscle traces flanking the notochord as valve structures, and misinterpretation of gonads in a subventrally exposed specimen as gut contents.
Both its old age and anatomical characteristics suggest that Y. lividum was a very primitive chordate. The most recent common ancestor of this group was a free-swimming, Yunnanozoon-like filter feeder having the following plesiomorphic features: (1) body laterally compressed and lancet-shaped; (2) notochord large and extended anteriorly; (3) myomeres nearly straight; (4) gill slits few in number; (5) gonads asymmetrically metameric; and (6) endostyle present. A Yunnanozoon-like ancestor may have given rise independently to urochordates, cephalochordates, conodonts, and agnathans (primitive vertebrates).
From the Bulletin of National Museum of Natural Science, No. 10, pp. 257-273, December 1997. Taichung, Taiwan. Used by permission.
The fossil record of the earliest chordates is extremely sparse. The upper Lower Cambrian Emmonsaspis (Walcott, 1890) from Vermont, was regarded as a chordate by Resser and Howell (1938) and Brasier (1979) . The chordate hypothesis (Resser and Howell, 1938; Brasier, 1979) was based primarily on misinterpretation of the chevron-like arrangement of branches as chordate myotomes. Conway Morris (1993) recognized it to be an Ediacaran frond-like fossil. The chordate affinities of Emmonsaspis are rejected by the absence of notochord and any other features related to chordates (Conway Morris, 1993). Metaspriggina from the Middle Cambrian Burgess Shale (Simonetta and Insom, 1993) is erected to receive the only poorly-preserved specimen preserved with a series of anteriorly pointing V-like structures, that were interpreted as muscle blocks of a chordate (Briggs et al., 1994). The alleged turnicates from the Lower Cambrian (Zhang, 1985) should be a misinterpretation. Until recently, the only known Cambrian Pikaia from the middle Cambrian Burgess Shale (Walcott, 1911), has been widely accepted as a chordate (Conway Morris and Whittington, 1979; Briggs et al., 1994). Chen et al., (1995) identified a number of features in Yunnanozoon lividum indicating that this taxon, currently known from the lower Cambrian Yu'anshan Formation at Chengjiang (figs 1, 2, in Chen and Zhou in this volume), eastern Yunnan, is also a chordate. Y. lividum was first described by Hou et al. (1991), who regarded it as a metazoan of unknown affinities, and interpreted its notochord as a "cavity" and its gonads as a "series of rounded structures." Shortly after Chen et al. (1995) recognized Y. lividum as the oldest chordate, Shu et al. (1996a) argued that Y. lividum was most closely related to hemichordates. This hypothesis, however, was based on (1) misinterpretation of the gonads in a subdorsally compacted (then ventrally exposed) specimen as gut contents; and (2) misinterpretation of metamerically arranged muscle blocks as spiral structures related to the intestine. The consistent rod-like shape of the putative intestine indicates that it can not be a relic of the digestive tract. Rather, it is a relic notochord. Most of the specimens illustrated by Shu et al. (1996a) were subjected to a complex history of reworking and early diagenesis, and this fact evidently escaped their attention. In seeking to support their hemichordate hypothesis, Shu et al. (1996a) misinterpreted the secondarily flattened dorsal musculature as a dorsal fin.
Shu et al. (1996b) also erected a new taxon, Cathaymyrus diadexus, for a single specimen purportedly representing the earliest chordate. This specimen, collected from the lower Cambrian Yu'anshan Formation, at Ma'anshan, Chengjiang, is 22mm long and laterally sinuous, and is thus similar in size and degree of lateral flexibility to Y. lividum. The dorsal ridge, interpreted by Shu et al. (1996b) as a possible notochord, is actually a dorsal fold like that seen in Y. lividum. Additional similarities to Y. lividum include the presence of gill slits and the arrangement of the transverse structures. In short, then, C. diadexus is probably a junior synonym of Y. lividum.
The purpose of this paper is to provide a complete description of Y. lividum. Twenty specimens collected from various quarries in the Mount Maotian area were examined. In addition, we discovered a layer containing a dense population reworked Y. lividum in the Maotianshan Member at Ma'anshan (October 1996). These additional specimens were also figured in the present description and reconstruction. The body of most specimens displays the notochord as a pair of axial lines representing its margins. The dorsal musculature consists if 22-24 segmented divisions (M1-M24). Metamerically arranged black blocks, preserved in several specimens, show that the notochord was flanked both laterally and ventrally by the muscles. Several specimens in our collection display a ventrally located, slender dark line representing the alimentary canal situated below the notochord. As indicated by analysis of these specimens, the origin and diversification of chordates was an integral part of the Cambrian Explosion, an event marked by the sudden appearance of numerous metazoan phyla (Chen et al., 1991)
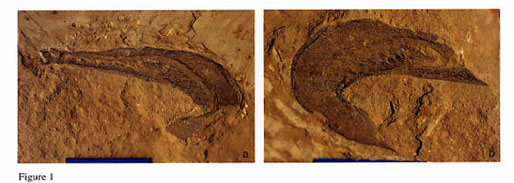
Yunnanozoon fossils are weathered in bluish gray, unlike other fossils in the Chengjiang fauna, which are reddish in color. This difference in weathering color probably reflects differences in original chemical composition (Hou et al., 1991). More specifically, the phosphate content of the soft tissues of Y. lividum may have been higher than that of other organisms. Because Y. lividum was soft-bodied, its remains were flattened into a thin film unless infilled with mud. The pharynx was the only body cavity susceptible to casting. Early phosphatization probably played a critical role in the preservation of morphological details. Fossilization of soft-tissues may have depended on a fine balance between phosphate-releasing decay and precipitation of phosphate by bacteria (Aldridge et al., 1993). The mode of preservation depended both on the chemical composition of the soft tissues and the early diagenetic environment, and thus varied considerably between different parts of the same individual and between individuals. The muscles above the notochord were segmented, and were divided by myosepta into 22-24 blocks. The myosepta are a prominent, well defined feature, and even preserve visible relief, with a darker color. As decay progressed the carcass became flattened, and phosphatization was enhanced by decay-induced phosphate release. The carcass was readily broken up if subjected to currents or other forms of reworking. The specimen shown in figure 1h of Shu et al. (1996a) has undergone post-mortem transport, and displays a detached muscle region. Shu et al. (1996a) misinterpreted this secondary flattening as an original anatomical feature. A number of tissues, including the gonads, the discs of the gill arches, and the endostyle, were decay resistant, and are preserved in a darker color as a mineral (presumably phosphate) replacement usually retaining some relief. The notochord apparently was not a favorable site for early phosphatization, but nevertheless it tends to be a prominent structure, represented in most specimens by a pair of axial lines. Y. lividum was vermiform and is generally preserved on its side, with strong post-mortem compaction and ventrally directed curvature. Subdorsally or dorsally compacted specimens are preserved in a laterally coiled (figs. 1-3; Chen et al., 1995, fig. 1a; Chen et al., 1996, fig. 285) or sinuous posture (Shu et al., 1996b, fig. 1), and represent individuals that were killed by sudden burial while swimming via lateral undulation.
Yunnanozoon lividum ( = Cathaymyrus diadexus Shu et al., 1996b) was small (20 to 40mm long) and laterally compressed, and had a nektonic mode of life similar to that of extant cephalochordates (fig. 4). The largest known individual, a laterally compacted, nearly complete specimen (fig. 5), was probably slightly longer than 39mm. The greatest height, which occurs at the mid-length of the animal, is 9.7mm. Specimen ELRC 52010 (fig. 2) represents a sublaterally compacted individual having a preserved length of 28mm. A split in the host matrix passes beneath the fossil, revealing both a compacted ventral margin and a compacted right flank. The specimen consists of the anterior part of an individual preserving a nearly complete pharynx and 18 muscule blocks. The original length of the animal was about 40mm, roughly equal to that of specimen ELRC 52001 (fig. 5). As indicated by the extent of reduction of both the height and width, the sagittal plane is tilted subvertically to the left side. The greatest height, as indicated by inspection of specimen ELRC 52001, originally was about 10mm, while the compacted height (indicated by the distance between the endostyle and the dorsal margin at the mid-length of the animal) is only 4mm, with an angle of tilt of about 66 degrees. The original width therefore was even less than 4mm. Specimen ELRC 52013a,b, (fig. 1), shows subdorsal compaction. The animal died in a laterally coiled posture, in a microturbidite mud layer. The fossil shows anticlockwise curvature in dorsal view, with increasing degrees of curvature posteriorly. A split in the host matrix passes beneath the fossil, exposing a ventral view from the left side. The original length of the animal was 38mm or slightly longer. The compacted height (as indicated by the distance between the mid-length of the paired gonads and the dorsal margin) is about 4mm. The original height was about 9mm. Y. lividum is rare in the Mount Maotian area. Thus far only twenty individuals have been identified among more than 10,000 soft-bodied fossils collected from this locality. It is much more abundant at Ma'anshan, where in 1996 we discovered a concentration layer. Here bedding planes are crowded with reworked specimens (fig. 6).
The body is fusiform, with the greatest thickness occurring at the mid-length of the body. The body form varies from thick (fig. 19) to slender (figs. 1,2). The cross section is narrow ellipse, as indicated by subvertically compacted specimens, with its height at least twice as great as the width. A low median dorsal fin is evident in specimens ELRC 52010 (fig. 2) and ELRC 53013 (fig. 1), possibly passing along the tail as tail fin and continuing along the ventral surface to the atriopore. The paired metapleural folds are present in most specimens in our collection. They occur along the anterior half of the ventro-lateral surface, diminishing posteriorly at immediate front of a notched structure interpreted as atriopore (figs. 12, 15).
The digestive system of Yunnanozoon is similar in its basic plan to that of living amphioxis, starting with anterior organ (fig. 7). At the posterior end of the anterior organ is a ring-shaped structure resembling a membranous velum in living amphioxis. The denticulated posterior surface of the ring is a possible attaching site of sensory tentacles, which guard the mouth and strain out large particle from the water current. The mouth is the circular opening within the ring. The large pharynx has seven pairs of branchial arches (B1-B7) and an endostyle in its ventral portion. At least two pairs of simple cone-shaped elements are present between B6 and B7 at the base of the branchial cavity. The alimentary canal starts behind the denticular organ and partitions longitudinally into esophagus, mid-gut and intestine with anus opening at M16.
Chen et al. (1995) interpreted the anterior organ as a snout. It is tunnel-shaped and opens to the exterior at its anterior end, and is usually filled with mud (fig. 1). Specimen ELRC 52002 (fig. 8) preserves the evidence that the anterior organ was flexible and capable of bending to the point where its anterior margin was directed ventrally (figs. 8, 16; Chen et al., 1996, fig. 284). The outer surface was papillose (fig. 8). The subquadratic structure within the snout, interpreted by Shu et al. (1996b, fig. 1g) as a proboscis, can also be interpreted as a mud infilling.
At the posterior end of the anterior organ is a ring-shaped structure about 0.7mm in diameter (figs. 3a, 12, 18), which is similar in basic morphology to a membranous velum in living Branchiostoma. The mouth represented by a circular opening, is situated in velum.
The pharynx lies in front of the visceral region and below the dorsal muscles. It was a spacious cavity susceptible to mud injection. Seven pairs of evenly spaced brachial arches occur in a number of specimens (Chen et al., 1995, figs. 1a-b, 2a-b, 3b). They extend postventrally from the sides of the notochord to a set of dark knobs located near the dorsal margin of the endostyle (figs. 9, 10, 18; Chen et al., 1995, fig. 3b). The knobs are solid, see also illustrations of knobs in specimens figured by Shu et al. (1996a, figs. 1b, 2d, 3b), but Shu et al. (1996a) misinterpreted them as gill pores. Each ramus of the branchial arches consists of about 20 dark bars and light interspaces that together resemble the striped, gill-bar mucocartilage in ammocoete larvae (Chen et al., 1995). The dark bars are disc-like, and are 0.24mm long and 0.18mm wide.
Two parallel lines extending along the base of the pharyngeal cavity, from the anterior end to just in front of the first gonad, represent the margins of the endostyle. They are parallel sided through major part of its length (having a width of 0.2mm), but taper posteriorly into a sharp end in its posterior part (fig. 19). The endostyle is a very prominent structure in specimens ELRC 25007 (fig. 9), ELRC 25011 (fig. 10), ELRC 25006 (fig. 11), and ELRC 25019 (fig. 18).
Most specimens show evidence of an axial, light gray tube flanked by a pair of linear lines (figs. 10a, b; Chen et al., 1996, fig. 282) that extend from the tip of the anterior organ (figs. 1, 8, 12; Chen et al., 1996, fig. 284), through the suprapharyngeal tissues (figs. 8, 12; Chen et al., 1996, figs. 284, 285), to the posterior end of the body (figs. 10, 13; Chen et al., 1996, figs 282, 283, 284). Chen et al. (1995) interpreted this tube as a relic of an originally fluid-filled notochord that underwent flattening shortly after burial. The notochord is flanked both laterally (figs. 11-13) and ventrally (fig. 1) by muscles that are preserved as metamerically arranged black bands. Shu et al. (1996a) misinterpreted the junctions of the muscle bands and the notochord sheath as a spiral structure related to the intestine. The notochord occupies about 25% of the total height of the trunk; this compares with 12.5-25% in extant agnathans and 40% in conodonts (Aldridge et al., 1993). The notochord is widest at the mid-length of the trunk and roughly parallel-sided along the anterior half, but tapers posteriorly to a sharp point (Chen et al., 1996, figs. 282-284). The notochord runs ventrally in most of the trunk, but lies close to the dorsal margin in the anterior 2/5 of the trunk. In this respect Y. lividum is similar to the conodont animal, in which the notochord occupies a dorsal position in the anterior region, a ventral position in the middle part, and a central position in the posterior part (Aldridge et al., 1993).
The recognition of myosepta is one of the most critical pieces of evidence for a euchordate affinity for Y. lividum (Chen et al., 1995). Specimen ELRC 52010 (fig. 2) preserves both the wrinkled epidermis and segmentally-arranged structures lying beneath the epidermis. The epidermis consisted of very soft tissues, and is preserved as a smooth surface in most of the body but is wrinkled locally to form striated structures. The facts that the striations extend forward and ventrally, rather than axially or transversely, and occur in discrete blocks or segments, indicate that they represent relic muscle tissue (fig. 2; Chen et al., 1996, fig. 280).
The myosepta are one of the most distinctive structures in Y. lividum, and are preserved in most specimens. They are darker than the rest of the body and show strong positive relief, extending subvertically from the dorsal margin of the trunk to the dorsal margin of the notochord. Indications of anatomical deformation caused by muscle contraction include: (1) sigmoidally curved septa (figs. 10, 12, 17); (2) narrow space between the septa (figs. 10, 12); (3) varied orientation of the septa, from perpendicular to oblique to the trunk axis (fig. 1).
The release of phosphate that accompanied the decay of the muscles may have promoted rapid fossilization. As indicated by examination of reworked material, the dorsal muscle region was the preferred site for early fossilization (fig. 6). The fossilized muscular region appears to have detached readily from the remainder of the trunk during physical reworking. The specimen illustrated by Shu et al. (1996b, figs. 1-h, 2) represents a carcass that has been flattened laterally and where its dorsal muscles fossilized early. Detachment of the rigid dorsal region from the trunk took place during transport. The carcass was finally buried with its middle section oriented dorsoventrally. Shu et al. (1996b) misinterpreted the secondary flattening of the dorsal muscles as an original feature.
A number of specimens preserve at least some evidence of metameric arrangement of black blocks on the sides of the notochord. In specimen ELRC 52011 (fig. 10) these black blocks can be traced along the entire length of the notochord. The metameric arrangement suggests that the black blocks represent ventral extensions of the myomeres. Shu et al. (1996a) interpreted a similar structure as spiral valves.
Several specimens in our collection preserve a dark tube representing the alimentary canal. The tube is situated below the notochord and extends from the rear margin of the endostyle within the posterior part of the pharynx.
Alimentary canal is the evident structure in ELRC 52002 (fig. 8), ELRC 52004 (fig. 12) ELRC 52011 (fig. 10) and ELRC 51014 (fig. 13). It can be arbitrarily subdivided into the esophagus, midgut and intestine. In ELRC 52002 (fig. 8) the esophagus is an anterior portion of the alimentary canal, represented by a narrow tube which opens anteriorly. The tube is situated below M4, extending posteriorly from rear margin of the endostyle and ending with a posterior constriction below M7. The midgut is situated within the posterior portion of pharynx, being the broadest portion of the alimentary canal, with its width diminishing both posteriorly and anteriorly. The posterior portion of the alimentary canal is intestine, represented by a well defined tube which tapers posteriorly (figs. 8, 10, 12, 13). An anus opens at point below M16 (figs. 8, 10, 13a). A spiral structure (figs. 8, 10, 12, 13) is visible through the entire length of the intestine. A similar structure is evident in a specimen illustrated by Shu et al. (1996a, fig. 1h), interpreted as a rectum.
The gonads are a prominent feature and are preserved in most specimens, with substantial relief. Thirteen pairs of gonads are arranged metamerically, with the first pair located beneath the fifth pair of muscle blocks. The gonads lie adjacent to the lateral sides of the intestine. The eight pairs of anterior gonads are considerably larger than the remaining pairs, which decrease in size posteriorly. In one subventrally compacted specimen (Shu et al., 1996a, fig. 1h), both the intestine and the gonads have been pushed together, within the outline of the notochord. Shu et al. (1996a, b) misinterpreted the gonads as contents of the intestine.
Denticular structure (Chen et al., 1995) is identified in several specimens (ELRC 52002, ELRC 52004 and ELRC 52013a; Hou et al., 1991, figs. 9a,b), represented at least by two pairs of simple conic elements (ELRC 52004) situated below M1 and anterior to esophagus. They are usually rusty in color, and retain relief (possibly mineralized).
A pair of elongated bands rimmed along the anterior half of the ventral surface of the body is here interpreted as the metapleural folds. The metapleural folds are a prominent feature and present in most specimens. In the anterior part of a subvertically compacted Yunnanozoon in ELRC 52013a (fig. 1a) both the right and left folds are present. In the anterior part of several laterally compacted specimens (ELRC 52002, ELRC 52004 and ELRC 52007) are two linear, axial lines, one situated above the endostyle and the other below it. The former may represent the upper margin of the trough-like structure at the base of the pharynx, while the other line probably represents the lower margin of the metapleural folds. Both lines extend longitudinally along the entire length of the pharynx (Chen et al., 1995, figs. 1-2).
A sublaterally compacted Yunnanozoon (ELRC 52003) shows the side view of a left fold, with indication of the fold at its broadest in its mid-length and diminishing posterioyly. Its posterior end is situated below M10 at a point in immediately in front of a notched structure. This notched structure is here interpreted as an atriopore. The atriopore can be traced by rear margin of the fold in a subvertically compacted specimen in ELRC 52010 (fig. 2).
The fins are absent in most specimens and apparently were susceptible to decay. Present in two specimens (figs. 1, 14; Chen et al., 1996, figs. 280, 285), from the dorsal to the muscle region, is a dark, elongate band here interpreted as part of the dorsal fin. The median dorsal fin is interpreted as passing posteriorly around the tail as tail fin, and continues forward anteriorly along the ventral surface to the atriopore as the ventral fin. The ventral fin is only partially preserved in ELRC 52003 (fig. 15) and ELRC 52010 (fig. 2).
Shu et al. (1996a) proposed a hemichordate affinity for Y. lividum, based on the hypothesis that this animal had a tripartite body. However this hypothesis is not supported by any intrinsic fossil evidence. The putative "subquadratic proboscis" (Shu et al., 1996a) may actually be a mud infilling of the snout, while the putative "stomochord," indicated by an obscure dark line within the tongue-shaped mud infilling of the snout (Shu et al., 1996a, fig. 1g), is probably part of the snout projecting from beneath the mud-filling.
The arguments of Shu et. al. (1996a) in interpreting the notochord as an intestine are based on misinterpretations of the metamerically arranged black blocks as "spiral structures" purportedly related to the intestine, and of the gonads as "spiral contents" in a vertically compacted specimen. The intestine hypothesis is clearly untenable. The notochord, preserved through sediment casting, is a stable feature. Most specimens preserve clear indications of axial lines similar to those representing the margins of the notochord in partially decayed Branchiostoma (Briggs and Kear, 1993). As indicated by examination of Y. lividum, the ancestral chordate possessed not only a large notochord, but also a pharyngeal cavity located beneath and directly adjacent to the notochord. This unique disposition, used by Shu et al. (1996a) as an argument against a chordate affinity for Y. lividum, actually has no bearing whatsoever on the question of its affinities.
Shu et al. (1996a) used the absence of complex muscle patterns in Y. lividum to argue against the hypothesis of a chordate affinity. However, one would expect the myomeres of primitive euchordates to be simple and nearly straight, evolving later into complex patterns such as the V-shaped musculature of Pikaia, conodonts, and extant cephalochordates.
Cathaymyrus was erected by Shu et al. (1996b) on the basis of a single specimen. It is here reinterpreted as a potential junior synonym of Yunnanozoon on the basis of the following similarities: (1) roughly similar size; (2) presence of a dorsal fold (interpreted as a notochord by Shu et al. (1996b); (3) identical arrangement in gill slits; (4) laterally compressed cross section; (5) Presumably identical numbers of subdivisions of musculature; (6) lateral sinuousity exhibit in some specimens; (7) same locality with roughly similar stratigraphic level.
The specimen studied by Shu et al. (1996b) is dorsally compacted, laterally sinusoidal, and represents a nearly complete individual slightly longer than 22mm. The anterior part is poorly preserved, with no anatomical details except for gill slits. Shu et al. (1996b) interpreted a narrow, mid-dorsal, ridge-like structure as a notochord. This feature is reinterpreted here as equivalent to the dorsal fold of Y. lividum. Shu et al. (1996b) correctly interpreted the transverse lines, some of which are sigmoidal or in geniculation, as myomeres. The complex pattern seen in dorsal view was produced by contraction of lateral muscle segments. Both the spacing and number of myomeres are roughly the same as in Y. lividum.
Although Branchiostoma can hardly be interpreted as a common ancestor of Chordata, it exhibits many features common to Yunnanozoon. We made some observations based on samples of B. belcheri collected from Fujian (China), and the major characteristics are figured (figs. 20-22) to facilitate the comparison with Yunnanozoon. The shared features between B. belcheri and Yunnanozoon include: (1) small body (rarely exceeding 5cm), laterally compressed and lancet-shaped, with a postanal tail; (2) a low, median dorsal fin; (3) a pair of metapleural folds ending behind the atriopore; (4) paired gonads; (5) notochord extending the entire body length; (6) a large pharynx with paired brachial arches; (7) digestive system consisting of a membranous velum and a mouth in the velum at its anterior end; (8) a trough-like endostyle; (9) digestive track partitioned into esophagus, midgut and hindgut. The differences, however are significant. Yunnanozoon features (1) a large subvetrally-situated notochord; (2) few gill slits; (3) nearly straight myosepta; (4) presence of denticulate structure within the basket cavity; (5) rigid endostyle; (6) gonads extending behind the atripore; and (7) spiral intestine.
In recognizing Yunnanozoon as a chordate, Chen et al. (1995) noted the following similarities between Y. lividum and cephalochordates: presence of metameric gonads, and presence of an anteriorly extended notochord. Chen (1995) argued that Y. lividum represents a basal chordate. The most recent common ancestor of Chordata may have resembled Y. lividum, being both nektonic and a filter feeder. Plesiomorphic chordate characters present in Y. lividum are: (1) bilaterally compressed body; (2) nearly straight myomeres; (3) large notochord, situated subventrally and extended anteriorly; (4) small number of gill slits; (5) asymmetrical disposition of gonads; (6) endostyle; and (7) dorsal fold and a pair of ventral folds. These features provide clues bearing on the phylogenetic relationships of the major groups of chordates.
It is here proposed that tunicates, cephalochordates, the conodont animal, and agnathans are sister groups derived separately from a common ancestor similar to Y. lividum. The small number of gill slits links tunicates, larval amphioxus, and lampreys (jawless fish) to the most recent common ancestor of Chordata. The presence of numerous gill slits in adult cephalochordates is an autapomorphy. The primitive nature of cephalochordates is indicated by the presence of the following plesiomorphic features: (1) notochord anteriorly extended; (2) small number of gill slits in the larval; (3) gonads metameric but laterally asymmetrical; and (4) existence of endostyle. The fact that cephalochordates have numerous gill slits casts doubt on hypotheses of a direct phylogenetic linkage to vertebrates. The oldest cephalochordate may be the middle Cambrian Pikaia, which has a narrow, dorsally situated notochord and abundant, V-shaped myomeres. A phylogenetic linkage to sessile tunicates is suggested by the presence of an endostyle and a small number of gill slits in the larva. The conodont animal and extant agnathans both have a large notochord, suggesting that they are deeply rooted. The presence of nearly straight musculature in Y. lividum indicates that this condition is the ancestral state, and that the V-shaped and other types of chordate musculature are therefore features secondarily derived.
The origin of the Phylum Chordata is a highly controversial problem, but the hypothesis that chordates are closely related to echinoderms is probably the most popular alternative. It is possible that sessile lophophorates gave rise to tunicates and that tunicates gave rise in turn to free swimming chordates, from which vertebrates evolved. Comparative morphological analysis suggests that chordates and echinoderms may have arisen from separate ancestors within the early lophophorates (Chen, 1995; Chen et al., 1996). Similarities between the early lophophorate Dinomischus and primitive echinoderms suggest that echinoderms may have been derived from a sessile lophophorate similar to Dinomischus. The ancestor of the chordates, on the other hand, may lie among worm-like lophophorates similar to Facivermis, which could have developed a notochord. Finally, the recent discovery of the crucial role of the Manx gene in the tail formation in tunicates (Pannisi, 1996; Swalla and Jeffery, 1996) may indicate that the origin of chordates could be traced back to a single genetic change.
This project was supported by funds from the Chinese Academy of Science, the National Geographic Society (Grant Numbers 4760-92, 5165-94, and 5670-96), the National Committee of Sciences and Technology, the National Science Council, the Government of Jiansu, and Mrs. Chunlan Wu. Reviews by Drs. Heyo Van Iten (Hanover College), and Xuanli Yao and Paul K. Chien (Univ. of San Francisco) are gratefully acknowledged. Mrs. Diying Huang and Bing Xu provided technical assistance.
Aldridge, R. J., Briggs, D. E. G., Smith, M. P. and Clarkson, E. N. K., 1993. The anatomy of conodonts. Phil. Trans. R. Soc. Lond. B, 340: 405-421.
Bliek, A., 1992. At the origin of chordates. Geobios, no. 25(1): 101-113.
Brasier, M. D., 1979. The Cambrian radiation event. In: House, M. R. (ed.). The origin of major invertebrate group, Systematics Association Special Volume 12. pp. 103-159. Academia Press; London.
Briggs, D., Erwin, D. and Collier, F., 1994. The fossils of the Burgess Shale. Smithsonian Institution Press.
Briggs, D. E. G. and Kear, A. J., 1994. Decay of Branchiostoma, implications for soft-tissue preservation in conodonts and other primitive chordates. Lethaia 26: 275-287.
Chen, J. Y., 1995. Contribution of the Chengjiang fauna to knowledge of coelomate explosive radiation. In: Chen, J. Y., Edgecombe, G. and Ramsköld, L. (eds.). International Cambrian Explosion Symposium (Programme and Abstract). pp. 7-9.
Chen, J. Y. and Erdtmann, D. B., 1991. Lower Cambrian Lagerstatte from Chengjiang, Yunnan, China: Insights for reconstructing early metazoan life. In: Simonetta, A. M. and Conway Morris, S. (eds.). the early evolution of Metazoa and the significance of problematic taxa. Cambridge University Press; Cambridge.
Chen, J. Y., Bergström, J., Lindström, M. and Hou, X. G., 1991. Fossilized soft-bodied fauna. National Geographic Research and Exploration 7(1): 8-19.
Chen, J. Y., Dzik, J., Edgecombe, G. D., Ramsköld, L. and Zhou, G. Q., 1995. A possible Early Cambrian chordate. Nature 377 (26): 720-722.
Chen, J. Y., Zhou, G. Q., Zhu, M. Y. and Yeh, K. Y., 1996. The Chengjiang biota - a unique window of the Cambrian explosion. National Museum of Natural Science; Taichun, Taiwan.
Conway Morris, S. and Whittington, H. B., 1979. The animals of the Burgess Shale. Scientific American 240: 122-133.
Conway Morris, S., 1993. Ediacaran-Like Fossils in Cambrian Burgess Shale-type Faunas of North America. Palaeontology 36 (3): 593-635.
Hou, X. G., Ramsköld, L. and Bergström, J., 1991. Composition and preservation of the Chengjiang fauna--Lower Cambrian soft-bodied biota. Zoologica Scripta 20: 395-411.
Garstang, G., 1928. The morphology of the Turnicata and its bearing on the phylogeny of the Chordata. Quart. Jour. Microscop. Sci. 72: 51-187.
Jefferies, R. P. S., 1971. Some comments on the origin of chordates. Journal of Paleontology 45: 910-912.
Jefferies, R. P. S., Lewis, M. and Donovan, S. K., 1987. Protocystites mencyensisÑa stem-group chordate (Cornuta) from the Middle Cambrian of South Wales. Palaeontology 30(3): 429-484.
Jefferies, R. P. S., 1981. In defense of the calcichordates. Zoological Journal of the Linnean Society 73: 351-396.
Pennisi, E., 1996. Tracing backbone evolution through a tunicate's lost tail. Science 274(15): 1082-1083.
Peterson, K. J., 1995. A phylogenetic test of the calcichordate scenario. Lethaia 28: 25-38.
Resser, C. E. and Howell, B. F., 1938. Lower Cambrian Olellus zone of the Appalachians. Bulletin of the Geological Society of America 49: 195-248.
Simonetta, A. M. and Insom, H., 1993. New animals from the Burgess Shale (Middle Cambrian) and their possible significance for the understanding of the Bilateria. Bolletino di Zoologia 49: 107-114.
Shu, D. G., Zhang, X. L. and Chen, L., 1996a. Reinterpretation of Yunnanozoon as the earliest known hemichordate. Nature 380(4): 428-429.
Shu, D. G., Conway Morris, S. and Zhang, X. L., 1996b. A Pikaia-like chordate from the Lower Cambrian of China. Nature 384: 157-158.
Strickberger, M. W., 1990. Evolution. Jones and Bartlett Publishers; Boston.
Swalla, B. J. and Jeffery, W. R., 1996. Requirement of the manx gene for expression of chordate features in a tailess ascidian larva, Science 274(15): 1205-1208.
Zhang, A. R., 1985. Fossil appendicularians in the Early Cambrian. Scientia Sinica, B.30: 888-896.
Walcott, C. D., 1890. The fauna of Lower Cambrian of Olenellus Zone. United States eological Survey, 10th Annual Reports (1891): 515-760.
Walcott, C. D., 1911. Middle Cambrian annelids. Cambrian Geology and Paleontology II. Smithsonian Miscellaneous Collections 57: 109-144.

Yunnanozoon lividum Hou, Ramsköld & Bergström, 1991; part (a) and counterpart (b) of a laterally coiled individual (ELRC52013, MN6).

Yunnanozoon lividum Hou, Ramsköld & Bergström, 1991; a lateral coiled individual (ELRC52010, MN5).

Yunnanozoon lividum Hou, Ramsköld & Bergström, 1991 (ELRC52001, MN6).
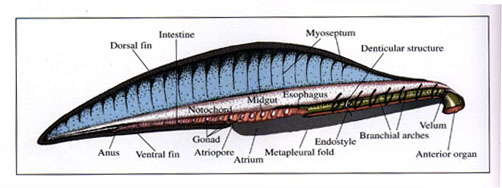
Anatomical interpretation of Yunnanozoon lividum Hou, Ramsköld & Bergström, 1991.
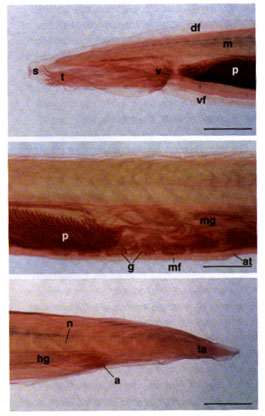
Light micrographs of a whole-mount specimen of Branchiostoma belcheri. Scale bar = 1 mm. [20] The anterior part of the body, showing the snout (s), tentacles (t), velum (v), dorsal fin (df), ventral fin (vf), anterior portion of the pharynx (p) with brachial arches, and V-shaped myomeres (m). [21] The middle part of the body, showing the posterior portion of the pharynx (p), midgut (mg), asymmetrically metameric gonads (g), atriopore (at), and metapleural folds (mf). [22] The posterior part of the body, showing the tail (ta), notocord (n), anus (a), and hindgut (hg).