August 1, 2004
Remember in last month's column I described a scene in which you were walking down a dark alley when you were suddenly attacked. Your immediate reaction was to wrench yourself free from your assailant and run for safety. In order for you to be aware of your attacker's grasp and be able to escape, you needed your neuromuscular system to be in proper working order. The sensory receptors for touch, pressure, and pain, would allow you to detect the presence of your attacker by sending nerve messages to the sensory region of the brain. In response to this insult the motor region of your brain would then send out nerve messages to your muscles in order for you to take evasive action.
In considering how nerve cells (also called neurons), allow us to be able to detect our environment, react, and interact within it, we considered three aspects of neuron function. The first was the initiation of a nerve impulse. In the scenario described above, this would be accomplished by the sensory receptors in the periphery and the motor strip in the brain. How some sensory receptors are able to convert physical stimuli, such as touch, pressure, and light waves, into messages that can be interpreted by the brain, will be dealt with in upcoming columns.
The second item was consideration of how the neuron is able to pass the impulse on from where it is first initiated to where it needs to go. Looking at the scene above this would entail explaining how the initiating sensory neuron or the motor neuron from the brain would be able to send the impulse down its axon. This was discussed in last month's column. I suggest that you may want to review "Wired for Much More than Sound-Neurons and how they Work-Part I-The Impulse" before continuing since everything from here on is built on the information from last month's column.
The final piece of the puzzle that needs explaining is how the neuron is able to transfer the impulse of information on to where it is needed for function. Once again, in considering the attack on your person in the dark alley as described above, once the impulse from the sensory receptor travels down the initial neuron, if it can't be transferred to other neurons so that the message can be sent to the brain, then nothing will have been accomplished.
Similarly, once the motor strip neuron initiates an impulse, the message needs to be able to be transferred to other motor neurons and eventually to muscle cells in order for proper evasive action to occur. If either the sensory or motor system is incapable of transmitting the proper messages then they will have failed in their mission and the very survival of a multi-system organism with a complex body plan will be held in the balance. The transfer of the nerve impulse to other nerve cells or muscle tissue is what this column will discuss.
Review
Recalling some of the specifics regarding neuron function from last month's
column:
The Synapse
Now if you've digested what I've described above and familiarized yourself
with the figures from last month's column, let's go ahead and discuss what happens
next. How exactly does the neuron transfer the impulse of information on to
another neuron or muscle cell? Remember now, the impulse is coming down the
axon toward its numerous nerve terminals. Across from the nerve terminals are
the nerve or muscle cells that are waiting for the message. In between them
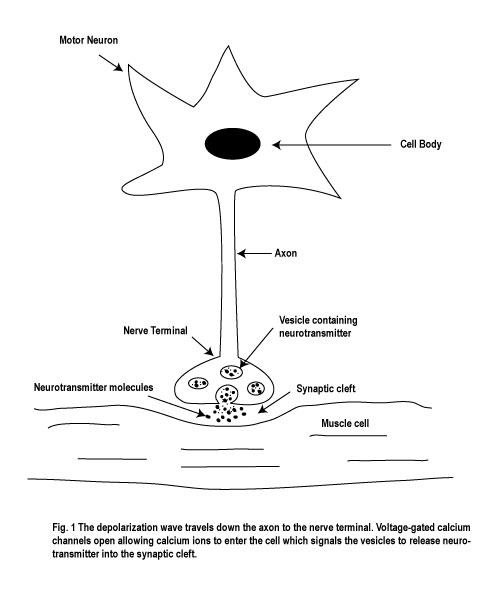
is a space called the synaptic cleft, which is about 20 nm (nanometers) in width.
Think of it like the English Channel, except the distance between Dover and
Calais is now only about one millionth of an inch. (see Figure 1)

The axon bringing in the message is known as the presynaptic neuron and the membrane of the nerve or muscle cell receiving the message is called the postsynaptic membrane. Within the nerve terminals of the presynaptic neuron are numerous synaptic vesicles that contain chemicals known as neurotransmitters. Many different neurotransmitters exist within different neurons. For example, acetylcholine is the neurotransmitter that is released by motor neurons in order to activate skeletal muscle cells. And dopamine, serotonin, norepinephrine, and glycine, are examples of neurotransmitters that are involved in synaptic transmission in the central nervous system.
Some of these neurotransmitters attach themselves to receptors that directly affect ion channels on the post-synaptic membrane. Others attach themselves to membrane bound enzymes which when activated causes an intracellular rise in a messenger chemical which ultimately results in a change in post-synaptic cell metabolism For the purposes of this column I will be focusing on the former mechanism only.
The actual release of the neurotransmitter is dependent on the change in the membrane potential of the pre-synaptic neuron. As the depolarization wave enters the nerve terminal, this activates voltage-gated Ca++ (calcium) ion channels which open up, allowing Ca++ ions to enter the cell. The elevation of Ca++ ions into the nerve terminal then acts as a signal and causes the synaptic vesicles to release their contents of neurotransmitters into the synaptic cleft. (see Figure.1).
As noted above, one common example of how neurotransmission takes place is that the neurotransmitter moves across the synaptic cleft and binds to specific receptors on the post-synaptic membrane which opens specific ion channels. These are known as chemical, or ligand-gated, ion channels (see Figure.2).

This usually causes the membrane potential of the post-synaptic cell to change by becoming more or less negative. For example, the neurotransmitter may be able to activate Na+ ion channels, allowing Na+ to enter the cell, and the post-synaptic membrane may start to depolarize (become less negative). If the post-synaptic cell is a neuron and enough activation occurs, this may result in that neuron totally depolarizing and sending the impulse down its axon on to another nerve or muscle cell. If the post-synaptic cells are muscle cells, enough activation may result in depolarization of the muscle membrane eventually resulting in contraction of the muscle. (the way this happens will be discussed in next month's column).
Similarly, the neurotransmitter released by the pre-synaptic neuron may bind to receptors on the post-synaptic neuron which open up negatively charged chloride (Cl-) ion channels instead of positively charged Na+ ion channels. Since the concentration of chloride outside of the cell is much higher than inside the cell, this will result in Cl- entering the cell through the opened ion channels resulting in the post-synaptic membrane becoming more negative and therefore rendering it less likely to be depolarized. This action will have, in effect, allowed the pre-synaptic neuron to have inhibited the post-synaptic cell by way of its neurotransmitter release into the synaptic cleft.
So one can readily see that neuromuscular function at the biomolecular level is indeed very complex. Specific pre-synaptic neurons secrete specific neurotransmitters into the synaptic cleft at the signal of Ca++ ions entering the nerve terminals through voltage-gated Ca++ ion channels upon depolarization of the cell membrane.
The neurotransmitter then crosses the synaptic cleft and locks on to specific chemical-gated ion channels which causes them to open and allow the specific ions to enter the post-synaptic cell resulting in either excitation or inhibition. But there's still more to consider. Let's look at what happens when a motor neuron sends a message to muscle tissue and tells it to contract.
Acetylcholine and Neuromuscular Transmission
Acetylcholine is the neurotransmitter for neuromuscular function. When it is
released by a motor neuron it is taken up by the acetylcholine receptors on
the postsynaptic membrane of the muscle cell. (fig.1) By locking onto these
chemical-gated positive ion channels (see fig. 2), this allows positive ions
like Na+ to enter the muscle cell. This leads to the depolarization of the muscle
membrane which eventually results in muscle contraction. So one can now see
how the nervous system is able to directly control muscle function.
However, it is important to recognize that the duration of the effects of acetylcholine on the muscle cell is limited by three main factors:
One of the key aspects of neuromuscular function is control.. If you turn something on, you also have to be able to turn it off in order to maintain control. Without the ability to limit the effects of acetylcholine by way of enzymatic breakdown, re-uptake by nearby tissue, and diffusion away from the myoneural synaptic cleft, this would allow acetyl-choline to have an unduly prolonged effect.
What do you suppose would result from this excessive activity of acetylcholine on muscle tissue? Think about it. Once the acetylcholine has been released, it will cause depolarization of the muscle membrane and the muscle will contract. What if the acetylcholine keeps hanging around and won't go away?
Initially the muscles would continue to contract without the ability to relax. This would eventually result in generalized muscle cramping, spasms and seizures. Then, after that, generalized paralysis would occur because the muscle cell membrane, under the effects of prolonged acetylcholine activity, would not be able to repolarize in readiness to allow for renewed contraction.
Without acetylcholine, its chemical-gated receptors on the postsynaptic muscle cell membrane, and the ability to limit its effects, particularly by acetylcholinesterase activity, the body would not be able to sustain any neuromuscular function and we would die.
Let's look at a few examples of medical illnesses or situations that directly result from defective neurotransmitter release, reception and breakdown.
Pathophysiology of Neuromuscular Dysfunction Resulting in Death
Botulism: a condition in which bacteria form a neurotoxin that blocks the
release of acetylcholine thereby resulting in progressive paralysis and death.
This poisoning syndrome demonstrates what would happen if acetylcholine did
not exist: death!!
Curare (arrow poison): neuromuscular blocker that attaches itself to the chemical-gated receptor of the ion channel on the plasma membrane of the muscle cell without stimulating it. This renders the ion channel being incapable of activation by acetylcholine, resulting in paralysis and death. This lesson demonstrates what would happen if the acetylcholine receptors on the post-synaptic muscle membrane did not exist: death!!
Acetycholinesterase inhibitors (e.g. insecticides): toxic exposure to these agents results in the muscle membrane being continuously depolarized as described above. This, at first, excites the neuromuscular system, and then paralyzes it, resulting in death. This shows what would happen if adequate supplies of acetylcholinesterase did not exist: death!!
Myasthenia Gravis: This is an auto-immune disease in which the body makes antibodies that attach to the acetylcholine receptors on the muscle cell membrane, destroying them, or rendering them incapable of activation. This results in progressive weakness and eventually death. This chronic disease once again demonstrates for us the importance of having an adequate supply of functioning acetylcholine receptors on the post-synaptic muscle membrane and what happens without them: death!!
Questions for Macroevolution
I hope that you can now understand how a neuron can send an impulse down its
axon and release a neurotransmitter from its nerve ends which can directly affect
another neuron or muscle cell resulting in a necessary function for the body.
Given this understanding and the added information afforded by the pathophysiology
of neuromuscular dysfunction resulting in death, let us consider the theory
of macroevolution, with its step-by-step mechanics, in order to see if such
a theory makes any sense.
To the questions from last month's column which involved:
We now add:
All nine of these components are necessary for properly controlled neuromuscular function, and in effect, for our survival on earth. If this system developed one step at a time, by small changes, as macroevolution proposes, then one must ask oneself which component came into existence first, what purpose did it serve that organism without the rest, and which component came next until the system as we know it existed?
Simply by looking just at acetylcholine, its metabolism, and how it exerts its effect, one can readily see that this would seem to be an impossible task. Medical science can show conclusively that not only is acetylcholine absolutely necessary for proper neuromuscular function, but so is the acetylcholine receptor on the postsynaptic muscle membrane. In addition, because the effect of unopposed acetylcholine on the postsynaptic muscle membrane has been demonstrated to render it in a state of constant depolarization and thereby prevents continued neuromuscular transmission, acetycholinesterase, the enzyme that limits acetylcholine's activity within the synaptic cleft, has also been demonstrated to be absolutely vital for proper neuromuscular function .
It is evident that all three of these factors must have come into existence at the same time in order to allow for the proper function of nerves and muscles which affords us the ability to survive on earth. Macroevolution must explain, not only how an organism could have developed the ability to produce acetylcholine and its controlled release mechanism within the motor neuron, but also what useful purpose it may have served without the presence of both, acetylcholine receptors on the ion channels of the post-synaptic cells, and the enzyme acetylcholinesterase. Think about it! How could controlled neuromuscular action have taken place in such an organism to allow for survival, knowing what we know about how this system actually works?
So! Do these facts point the mind to accept macroevolution and its step by step mechanics as being an absolute truth of Nature? Or do they point us to the distinct possibility that this system just may have been intelligently designed? Which is more reasonable? Which is more plausible and consistent with our own experience and understanding of how complex systems come into existence and how they function?
Just consider the myriad of human invention. Yet, the way in which one's own body is able to be alive here on earth points to a complexity and beauty that is infinitely greater than all of human creativity.
As a physician, I am daily called upon to make decisions that affect my patients, based on numerous bits of information that ultimately requires analyses. After considering the information set before me, and provided for your perusal in this and previous columns, and more to come, for my part, I choose the latter diagnosis of intelligent design, until such time that science can adequately answer the questions that I have raised here in these columns. For who, on encountering the proverbial watch in the field, would for even a moment, consider that it had come about by the random forces of nature? Not many people I venture. Yet so many people have come to uncritically believe in macroevolution despite the contradicting evidence contained within the biomolecular basis of life and the pathophysiology of disease, dysfunction and death.
Next month, as promised, we'll look at what happens to make muscles contract. If you've understood these last two columns, you're already half-way there. See you then in: Wired for Much More than Sound: Part III: Muscle Cells and How they Contract
Dr. G.
Howard Glicksman M. D. graduated from the University of Toronto in 1978. He practiced primary care medicine for almost 25 yrs in Oakville, Ontario and Spring Hill, Florida. He recently left his private practice and has started to practice palliative medicine for a Hospice organization in his community. He has a special interest in how the ethos of our culture has been influenced by modern science’s understanding and promotion of what it means to be a human being.
Copyright 2004 Dr. Howard Glicksman. All rights reserved. International
copyright secured.
File Date: 8.01.04